Second Question
We now consider the effect on our
calculations when we factor in surface tension.
The formula for the surface tension
of a liquid whose surface may be defined by the curve u(x) is
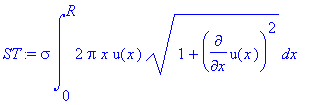
where σ is a constant which is a function
for temperature. For water, the value is
σ = 0.07 N/m.
Surface tension is a source of
energy in this system, so we add it into our total energy equation, giving us
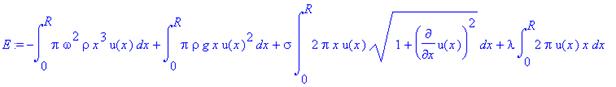 .
.
As
before, we minimize our function by substituting the integrand
![]() ,
,
where z = u(x) and p=u’(x), into the
Euler-Lagrange equation
![]() ,
,
giving us
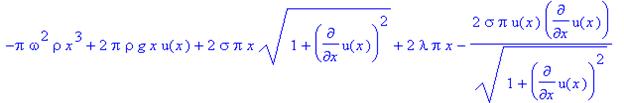
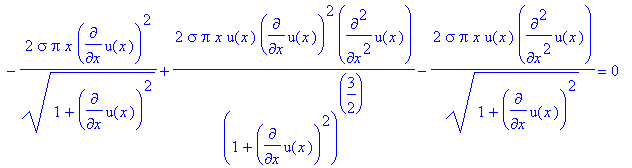
after we substitute u(x) and u’(x) back in for
z and p.
However, we cannot solve the equation for
u(x) in this form because it is nonlinear, so we’re not guaranteed a long-term
numeric solution. We solve this problem
by approximating u(x) with a power series
![]() ,
,
which, substituting into our solution from the
Euler-Lagrange equation, gives us
![]()
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image018.gif)
![]()
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image022.gif)
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image024.gif)
![]()
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image028.gif)
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image030.gif)
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image032.jpg)
![eq6 := -pi*omega^2*rho*x^3+2*pi*rho*g*x*(a[0]+a[1]*...](2ndques_files/image034.gif) .
.
We
can simplyify this, but the result is an excessively long equation. We can simplify
this equation by taking the fifth order
But now we have seven unknowns—the values
a0 through a6—which we need to solve for. We start by observing that since the original
Euler Lagrange equation is equal to zero, then so is the
Taking
our remaining values a0 and a2 through a6, we
can solve for the latter to get
which we then substitute back into our power
series to give us an approximation of u(x):
![u_1(x) := a[0]+.5156534608e-10*(4848217243.+.438199...](2ndques_files/image061.jpg)
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() .
.
Our
coefficients are in terms of a0, and we cannot solve them any
further. Thus, we must rely on our
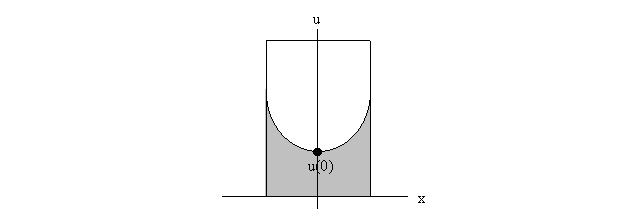
experimental data to satisfy the value of a0. For each trial, we set a0 equal to
the experimental value closest to 0 because a0 is the initial condition u(0) by
definition, and u(0) should theoretically fall at the lowest point of the
rotating water, as seen below on the diagram of a cross section of the system.
A
complete analysis of the effect of surface tension on our calculations is
included in the Data Analysis and Conclusions section.
![eqns := {a[2] = .5156534608e-10*(4848217243.+.43819...](2ndques_files/image037.jpg)
![eqns := {a[2] = .5156534608e-10*(4848217243.+.43819...](2ndques_files/image059.gif)